2025-11-21
SITC 2025 | Single Intravenous Infusion Leads to Complete Eradication of High-Grade Glioblastoma in 4 Weeks
he 40th Annual Meeting of the Society for Immunotherapy of Cancer (SITC) will officially kick off in Maryland, USA, from November 7 to 9, 2025. As one of the world's largest and most prestigious international conferences focused on cancer immunotherapy, the SITC Annual Meeting is dedicated to advancing the science and application of cancer immunotherapy, discovering breakthroughs, and improving outcomes for all cancer patients.
The latest clinical breakthrough of Junsai Bio's GC101 has been included in the meeting. A patient with grade IV glioblastoma achieved complete tumor eradication just 4 weeks after a single intravenous infusion of GC101 Tumor-Infiltrating Lymphocyte (TIL), and has maintained tumor-free survival for over 20 months to date. This breakthrough brings new hope for the treatment of such advanced malignant tumors and will be shared with global experts through a poster presentation and online format.
Poster Details
- SITC 2025 Anniversary: 40 Years of Driving Breakthroughs in Cancer Immunotherapy
- Date: Pre-Conference Programs (Nov. 5-6); Annual Meeting (Nov. 7-9)
- Venue: National Harbor, MD
- Abstract Number: 536
- Abstract Title: Phase 1 Trial of Tumor-Infiltrating Lymphocyte (TIL) Therapy in Recurrent Glioblastoma: A Single-Center Experience Using Cells Derived from Primary Surgical Specimens
- Principal Investigators: Professors Chen Yanming and Lan Qing
- Research Institution: The Second Affiliated Hospital of Soochow University
- Presentation Time: Saturday, November 8, 2025, 10:00 A.M.-6:35 P.M. (local time)
- Official Poster: Available on Junsai Bio's official website starting November 7
As one of the most common primary intracranial tumors, glioblastoma is regarded as a "tough nut to crack" in the field of oncology due to its high malignancy and strong heterogeneity. Traditional treatment combining surgery, radiotherapy, and chemotherapy not only struggles to eradicate lesions but also causes severe side effects. The median survival time of patients is 6-10 months, with a 5-year survival rate of less than 10%. More challenging still, the blood-brain barrier (BBB) acts as a "natural barrier," blocking most drugs and severely compromising therapeutic efficacy.
Based on Junsai Bio's independently developed DeepTIL™ technology platform, its innovative segmented process achieves key technological innovations. Tumor tissue is obtained during the patient's initial surgery to pre-process and preserve TIL seed cells. Upon recurrence, the cells are rapidly thawed, expanded, and infused—shortening waiting time while retaining highly active tumor-killing cells.
Conventional IL-2-dependent TIL therapy for glioblastoma has certain limitations: on one hand, it is difficult to culture clinical-grade TIL cells from "cold tumors"; on the other hand, IL-2 injection may increase the risk of severe adverse events such as cerebral edema and intracerebral hemorrhage.
Junsai's GC101 innovative TIL therapy eliminates the need for lymphodepleting preconditioning and IL-2 injection. It can autonomously cross the blood-brain barrier after intravenous infusion to precisely target and kill tumors, offering the advantages of rapid onset, long-lasting efficacy, and minimal side effects. This breakthrough in glioblastoma treatment follows significant progress of GC101 in other cancer types including pancreatic cancer, melanoma, lung cancer, and gynecological tumors (click to review case reports), and is expected to completely transform the treatment paradigm for glioblastoma.
From Despair to Hope: The Countdown of Glioblastoma
In early 2023, Mr. W was diagnosed with glioblastoma (IDH wild-type, WHO grade IV, EGFR-amplified)—one of the most malignant and treatment-refractory subtypes of glioblastoma. At the moment of diagnosis, an invisible hand pressed the countdown on his life.
Despite undergoing high-risk brain tumor resection, post-operative temozolomide chemotherapy, and radiotherapy, his condition remained uncontrolled. He subsequently received two different targeted CAR-T therapies with more than 10 intratumoral injections combined with tumor treating fields (TTF) therapy, but none could sustainably halt tumor progression.
Patient's Treatment History
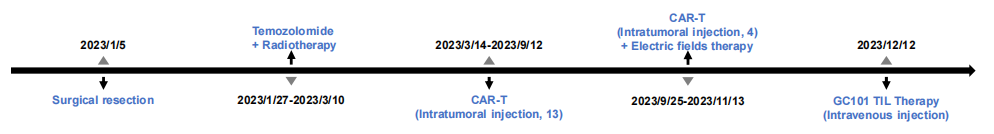
In late 2023, when Mr. W and his family were in despair, a turning point emerged. Mr. W successfully enrolled in the GC101 clinical trial and received TIL cell infusion (cells derived from surgical specimens collected one year earlier). Only 4 weeks after infusion, imaging showed complete eradication of the brain tumor, with efficacy evaluated as Complete Response (CR). No recurrence has been observed since, and he has maintained tumor-free survival for over 20 months. Today, Mr. W has long been free from hospital beds, reborn to return to a normal life.
Patient's MRI Changes
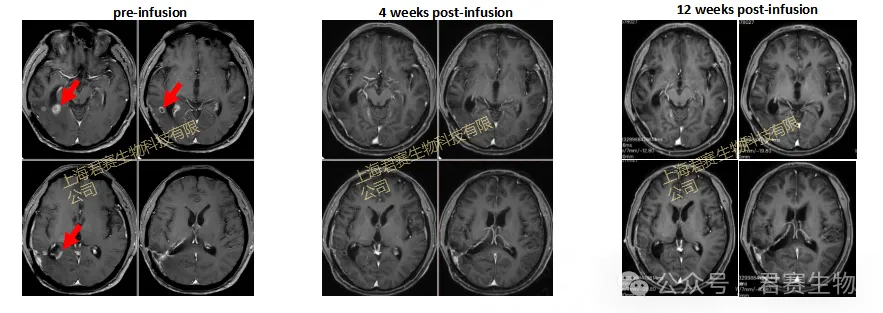
- Pre-infusion (December 2023): Sustained tumor progression
- 4 weeks post-infusion: First imaging evaluation, complete tumor eradication
- 12 weeks post-infusion: Second imaging evaluation, maintained tumor-free status
To date, this case not only sets a new survival record for recurrent glioblastoma but also validates the long-term efficacy of TIL therapy with robust clinical data. It further provides critical clinical evidence for the treatment strategy of "early harvesting and TIL infusion upon recurrence," bringing hope to countless patients with advanced solid tumors facing desperate situations.
References
- https://pubmed.ncbi.nlm.nih.gov/38769543/
- https://ascopubs.org/doi/pdf/10.1200/JCO.2024.42.16_suppl.5552
- https://www.brainmed.com/info/detail?id=49249
